Nigeria has become the second country to authorise the use of a novel malaria vaccine.
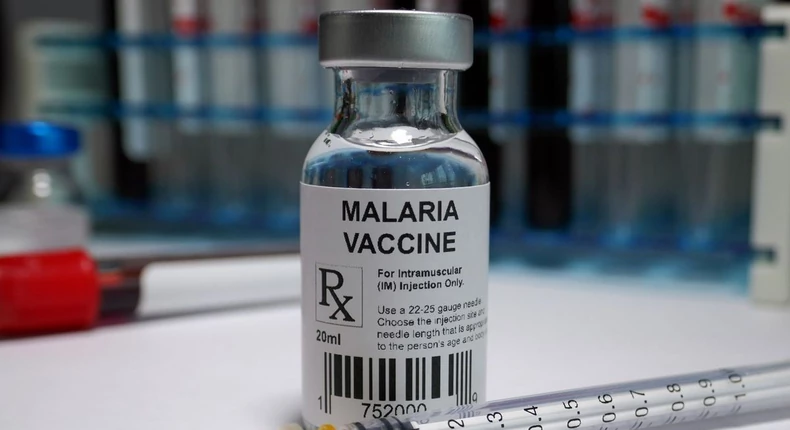
According to the Nigerian newspaper Punch NG, Prof. Mojisola Adeyeye, Director General of Nigeria's drug regulatory authority, NAFDAC, presented this information on Monday during a news conference in Abuja. Nigeria is now the second country to embrace the innovative malaria vaccine developed by the University of Oxford, following Ghana. Prof. Adeyeye recommends using the vaccination in children between the ages of 5 and 36 months to avoid the onset of clinical malaria. She noted that the nation anticipates receiving at least 100,000 doses of the vaccine in contributions shortly before the market authorization will begin making further arrangements with the National Primary Health Care Development Agency. "NAFDAC is granting registration approval for R21 Malaria Vaccine (Recombinant, Adjuvanted) manufactured by Serum Institute of India Pvt. Ltd. in accordance with its mandate as stipulated by its enabling law, NAFDAC Act CapN1, LFN 2004." According to the Agency's Drug and Related Products Registration Regulation 2021, the Marketing Authorization Holder is Fidson Healthcare Ltd," Prof. Adeyeye remarked. According to the professor, the R21 malaria vaccine dossier considerably conformed to the highest international standards against which the dossier was benchmarked. According to Prof. Adeyeye, the Joint Review Committee determined that the R21 malaria vaccine data was trustworthy and met the standards for efficacy, safety, and quality. Furthermore, it was found that the vaccine's known and potential benefits outweigh its known and potential dangers.
Category Local NewsNews Source https://africa.businessinsider.com/local/markets/nigeria-is-the-second-country-t
Date Posted 2 years ago